Research Focus
We take an interdisciplinary approach that combines proteomics technology with biological inquiries
Mapping the secretome
Discovering the role of the secretome of cells and tissue in disease
Cells in the human body communicate with each other constantly by sending signals that travel outside the cell. Some of these signals are in the form of soluble proteins, whereas others are enclosed in membrane-bound corpuscules called extracellular vesicles. The discovery of cell-to-cell signaling is challenging because the circulating plasma contains a mixture of molecules that originate from virtually all tissues in the body, making it difficult to disentangle where each signal comes from and where they go. Our lab is interested in using experimental and bioinformatics approaches to discover these cell-to-cell signals, as well as identify potential messengers of long-range endocrine signaling. Some of our works include:
Mapping the exosome contents of cardiac cell types
Exosomes are released from the cell through a specific cellular pathway that involves a sorting protein complex known as ESCRT. Exosome research has received intense attention in cardiovascular medicine in the past decade, spurred in part by the search for cell therapies that can help heal or rejuvenate the cardiac muscle after an injury such as a heart attack. Working with the Wu Lab at Stanford and the Chandy Lab at UWO, we are working to identify the micro RNA molecules that are packed inside the exosomes released from cells in the heart. Micro RNAs are an important class of nucleic acids in the cell that do not code for proteins but instead modulate the expression of other genes. Exosomes are known to contain multiple micro-RNAs that are also called exomirs (for exosomal micro RNAs). To discover the identities of the exomirs released by different types of cells in the heart, we are taking advantage of the power of induced pluripotent stem cells to differentiate into different cell types that compose the human heart, including cardiomyocytes, endothelial cells, and fibroblasts. Surprisingly, each cell type releases exosomes that contain very different exomirs that reflect the different biology of these cell types. For instance, cardiomyocytes release exomirs that are known to be expressed primarily in the heart, whereas fibroblasts which are present in different organs in the body release exomirs that are ubiquitously expressed. This difference is so pronounced in fact that sampling the micro RNAs in a plate of cell culture and be able to guess the cell type composition in the dish. For example, detecting one type of exomir (miR-302a) in the tissue culture medium is a sensitive indication that there are undifferentiated iPSCs present in a dish of cardiomyocytes. This could lead to convenient assays to rule out potential contamination of undifferentiated cells, which is a serious concern for producing cardiomyocytes for potential human use, since these undifferentiated cells may have the potential to develop into unwanted cell types or even tumors.
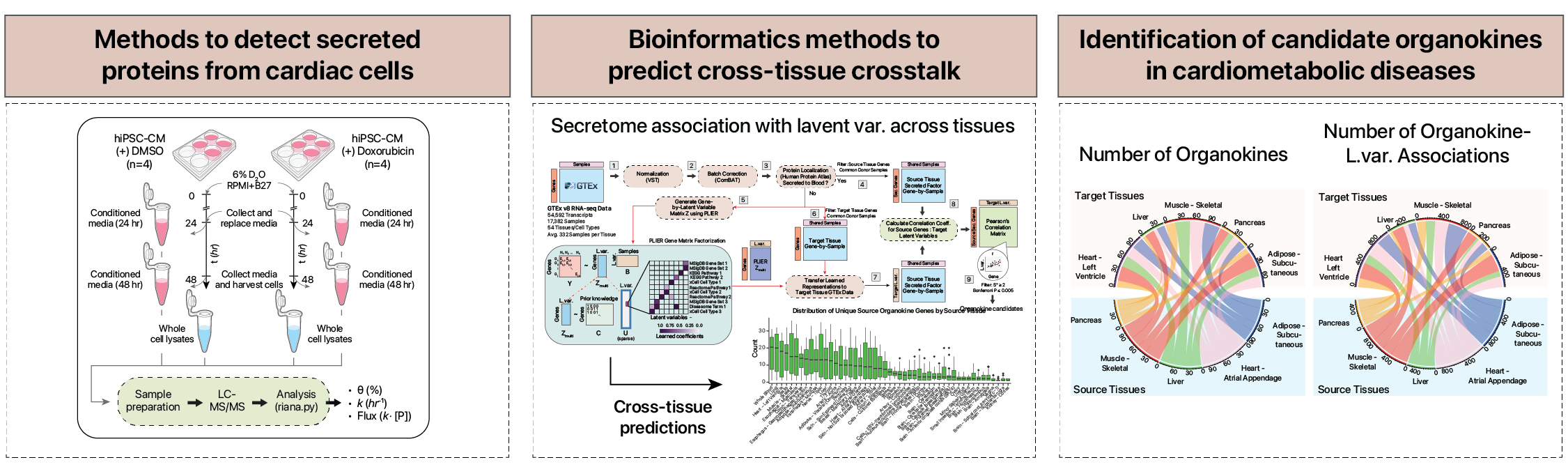
Protein secretome from the heart to other tissues
Besides its vital role in circulation, the heart has an important secondary function as an endocrine organs. A well known example is the natriuretic peptides released from cardiac cells, which can exert a wide range of physiological effects on other tissues such as the kidneys to control blood volume and pressure. In recent years, more and more proteins and peptides that are potentially released from the heart have been found in experiments, some of which may be important in human aging processes. In a recent paper, we asked how many of these heart-released proteins, or "cardiokines" there could potentially be that affects aging in other tissues of the body. To do that, we are analyzing large scale human data on heart-expressed transcripts as well as potential proteins in the human proteome that are released to the blood. A correlation analysis found that hundreds of cardiac transcripts could potentially change in expression levels as humans age, which could hint at important aging-related functions. More importantly, not every such gene has the same age-associated changes, with some that show different abundance only in particular tissues but not others, suggesting they could serve as a heart-specific aging signal. In more recent work, we have developed a new method, termed SALVE, to predict cross-tissue correlation of secretome-coding genes in a source tissue and a gene module in a target tissue within GTEx data. This approach may be useful for finding endocrine signal candidate between two human organs
Team Members
Related Publications
SALVE: prediction of interorgan communication with transcriptome latent space representation.
Pavelka, Jay , Voong, Calvin K , Schaal, Peyton , …
Am J Physiol Heart Circ Physiol (2025)Defining the Roles of Cardiokines in Human Aging and Age-Associated Diseases.
Srivastava, Himangi , Pozzoli, Marina , Lau, Edward
Front Aging (2022)Atlas of Exosomal microRNAs Secreted From Human iPSC-Derived Cardiac Cell Types.
Chandy, Mark , Rhee, June-Wha , Ozen, Mehmet O , …
Circulation (2020)Last updated: January 27, 2026


